Industry News Home > > News > Industry News
How to prepare Tris hydrochloric acid buffer
Tris is a weak base. At room temperature (25 ℃), its PKA is 8.1; the pH of Tris buffer is between 7.0-9.2.
The pH value of Tris base aqueous solution is about 10.5. Generally, hydrochloric acid is added to adjust the pH value to the required value to obtain the buffer solution of the pH value. But at the same time, we should pay attention to the influence of temperature on the pKa of Tris. Several common Tris hydrochloric acid buffer configuration methods are as follows:
Mix 50ml of 0.1M Tris solution with X ml of 0.1M hydrochloric acid, and dilute to 100ml with water
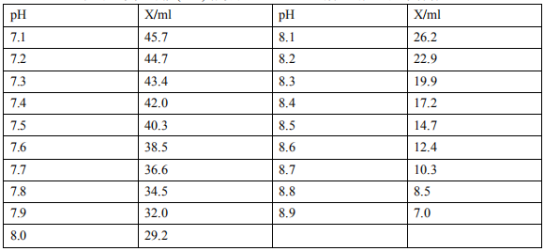
Tris molecular weight = 121.140.1m, 12.114g/l
Tris solution can absorb carbon dioxide from the air. Pay attention to tight bottle cap when using.







